Achievements
Research by medical students published in an English academic journal at the same time as an academic conference presentation: Risk of side effects from low-dose aspirin administered for primary prevention of cardiovascular events in type 2 diabetes patients decreases after three years of treatment.
A research group led by Naoko Masutani, a sixth-year School of Medicine Hyogo Medical University (Location: Nishinomiya City, Hyogo Prefecture; President: Keiichiro Suzuki), and Tsuyoshi Morimoto, professor and head Department of Data Science, analyzed the long-term effects of low-dose aspirin, which is administered for the primary prevention of cardiovascular events in patients with type 2 diabetes, on the incidence of side effects such as gastrointestinal symptoms, and found that the risk decreases three years after the start of administration.
The results of this research were presented orally at the 72nd Annual Meeting of the Japanese Society of Cardiology held in Sendai on September 29th, and published online in the American Journal of Cardiovascular Drugs on the same day. Simultaneous presentation at an academic conference and publication in an English academic journal is almost always the case for large-scale research by top-class researchers, but this has never been achieved by a School of Medicine student.
Key points of this study
●It has been revealed that the risk of side effects such as gastrointestinal symptoms caused by low-dose aspirin, which is administered for the primary prevention of cardiovascular events in patients with type 2 diabetes, decreases after three years of starting administration.
●It will be helpful for patients who are currently taking aspirin or are considering taking it in the future to decide whether to take it.
Research Overview
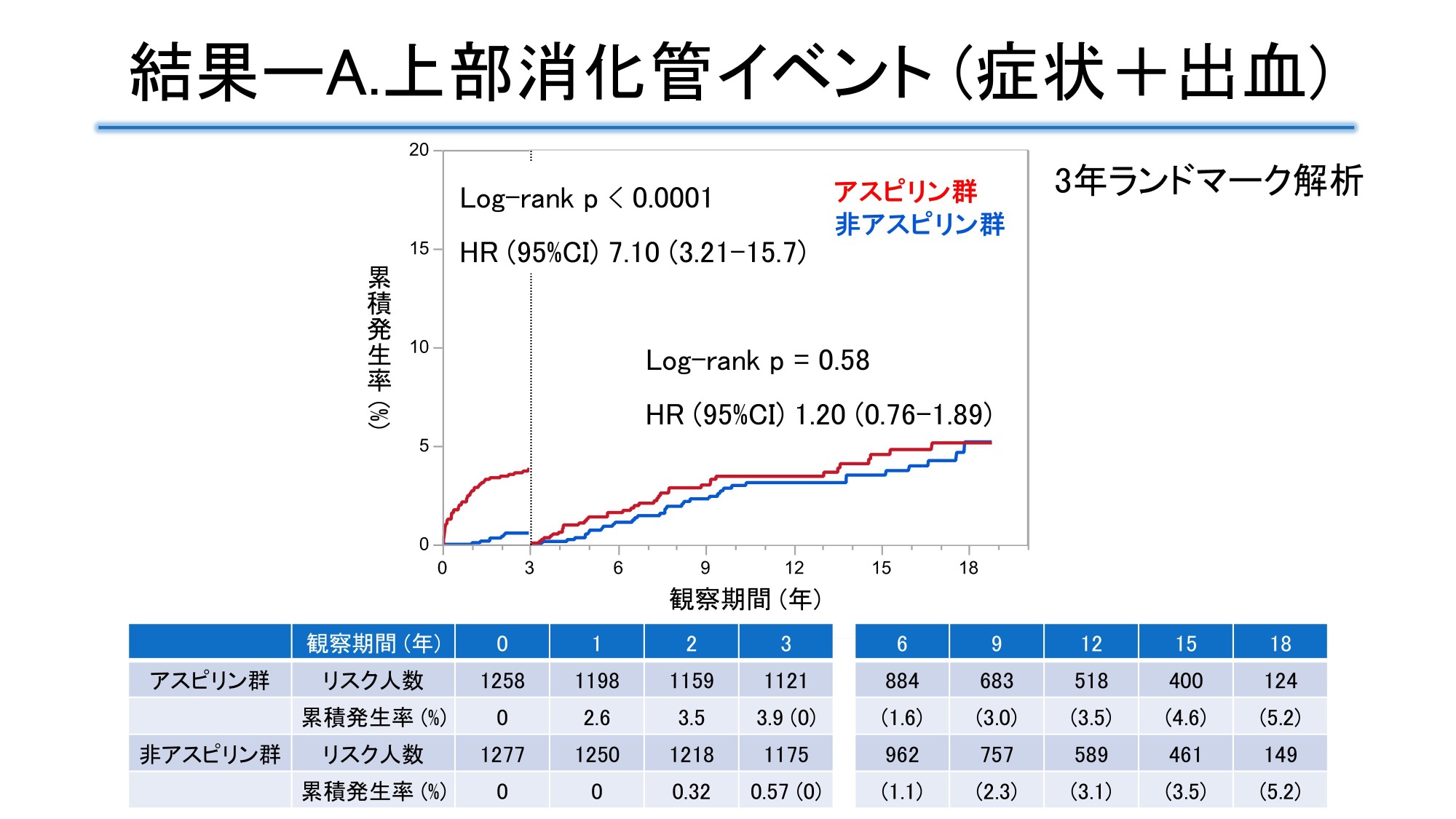
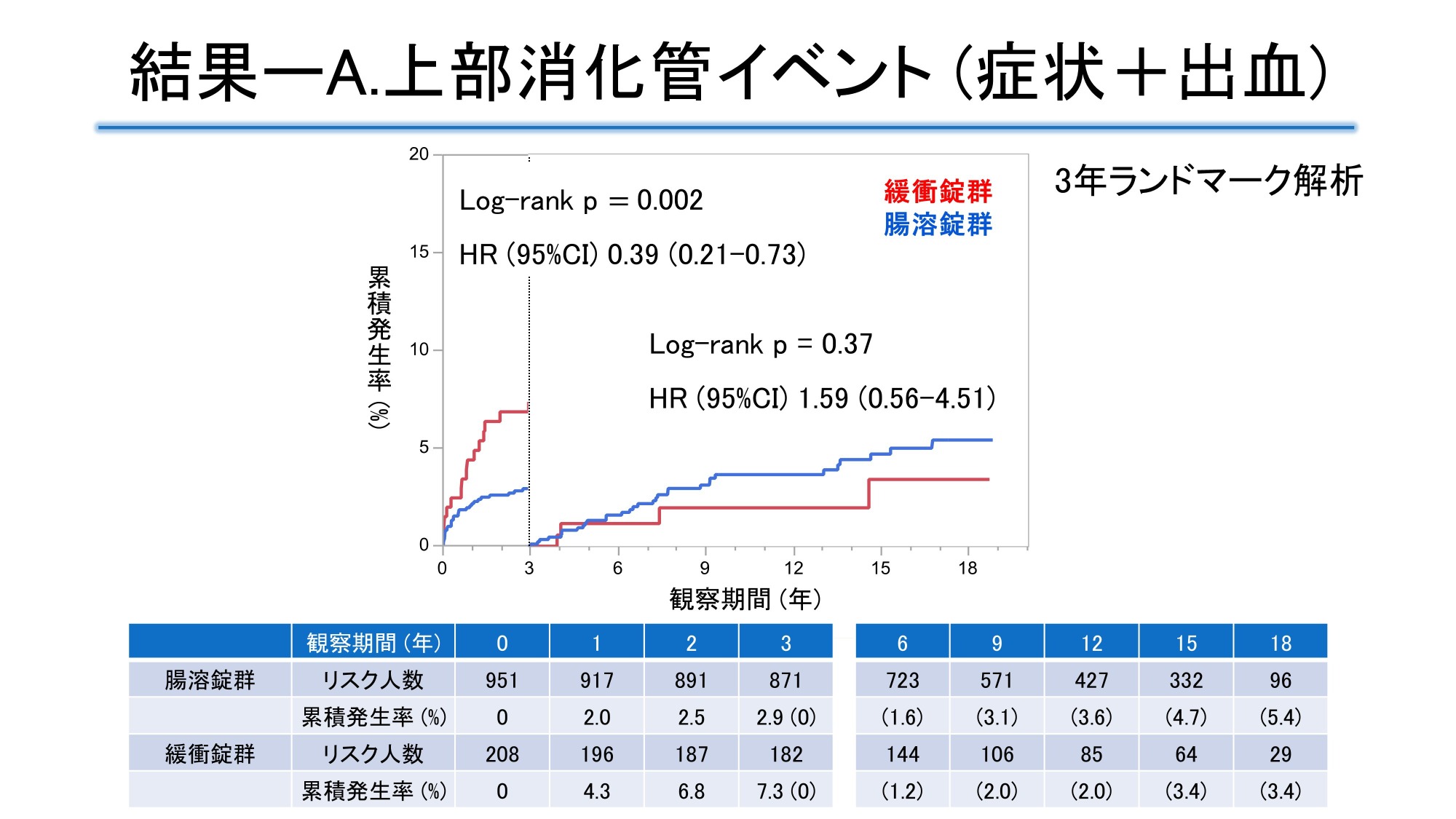
Using data from the JPAD trial, a randomized clinical trial of low-dose aspirin in patients with type 2 diabetes, and its follow-up study (median observation period 11.2 years), the incidence of upper gastrointestinal symptoms or bleeding within 3 years and beyond was examined in 1258 patients in the aspirin group (951 patients receiving enteric-coated aspirin tablets, 208 patients receiving buffered aspirin tablets, and 99 patients receiving unknown aspirin) and 1277 patients in the non-aspirin group. The cumulative incidence of upper gastrointestinal symptoms or bleeding was significantly higher in the aspirin group, with a hazard ratio (HR) [95% confidence interval (CI)] of 2.20 [1.52-3.18] in the aspirin group over the entire period. The risk in the aspirin group was significant within 3 years (HR 7.10 [3.21-15.7]), but attenuated beyond 3 years (HR 1.20 [0.76-1.89]). In the aspirin group, the adjusted HR for the enteric-coated tablet group within 3 years was 0.38 [95% CI 0.20-0.72], which was lower than that for the buffered tablet group.
These observations suggest that upper gastrointestinal symptoms and bleeding due to low-dose aspirin require careful attention for the first three years after starting treatment, but that the risk decreases after three years.
Research Background
Low-dose aspirin is widely used as a primary and secondary prevention for patients at high risk for cardiovascular events. Diabetic patients are particularly at high risk for cardiovascular events, and in the United States, 62% of diabetic patients aged 60 years or older take low-dose aspirin. However, low-dose aspirin is not recommended as a primary prevention for patients at risk of bleeding, a side effect of low-dose aspirin, and clinical epidemiology is required, particularly regarding the incidence and timing of gastrointestinal bleeding and gastrointestinal symptoms, which are the precursors to gastrointestinal bleeding.
Research Methods and Results
A post-hoc analysis of randomized clinical trials and follow-up studies was conducted on 2,535 patients (1,258 in the aspirin group and 1,277 in the non-aspirin group) enrolled in the Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes (JPAD) Trial, a randomized clinical trial that evaluated the primary prevention of cardiovascular events with low-dose aspirin in patients with type 2 diabetes. The patients were observed for up to 19 years from the start of enrollment in December 2002 to the final follow-up in July 2021, and the composite endpoint (upper gastrointestinal events) consisting of upper gastrointestinal symptoms (upper abdominal pain, nausea, vomiting, loss of appetite, etc.) and upper gastrointestinal bleeding, as well as upper gastrointestinal symptoms excluding bleeding, upper gastrointestinal bleeding, and all bleeding events were evaluated.
The cumulative incidence of each event was compared, and an analysis was also performed for the aspirin groups, dividing them into 951 patients in the buffered tablet group and 208 patients in the enteric-coated tablet group. Landmark analyses were performed, dividing the observation period into those within 3 years after randomization and those beyond 3 years, to estimate the cumulative incidence of each event and the hazard ratios (HRs) and 95% confidence intervals (95% CIs) using the Cox proportional hazards model.
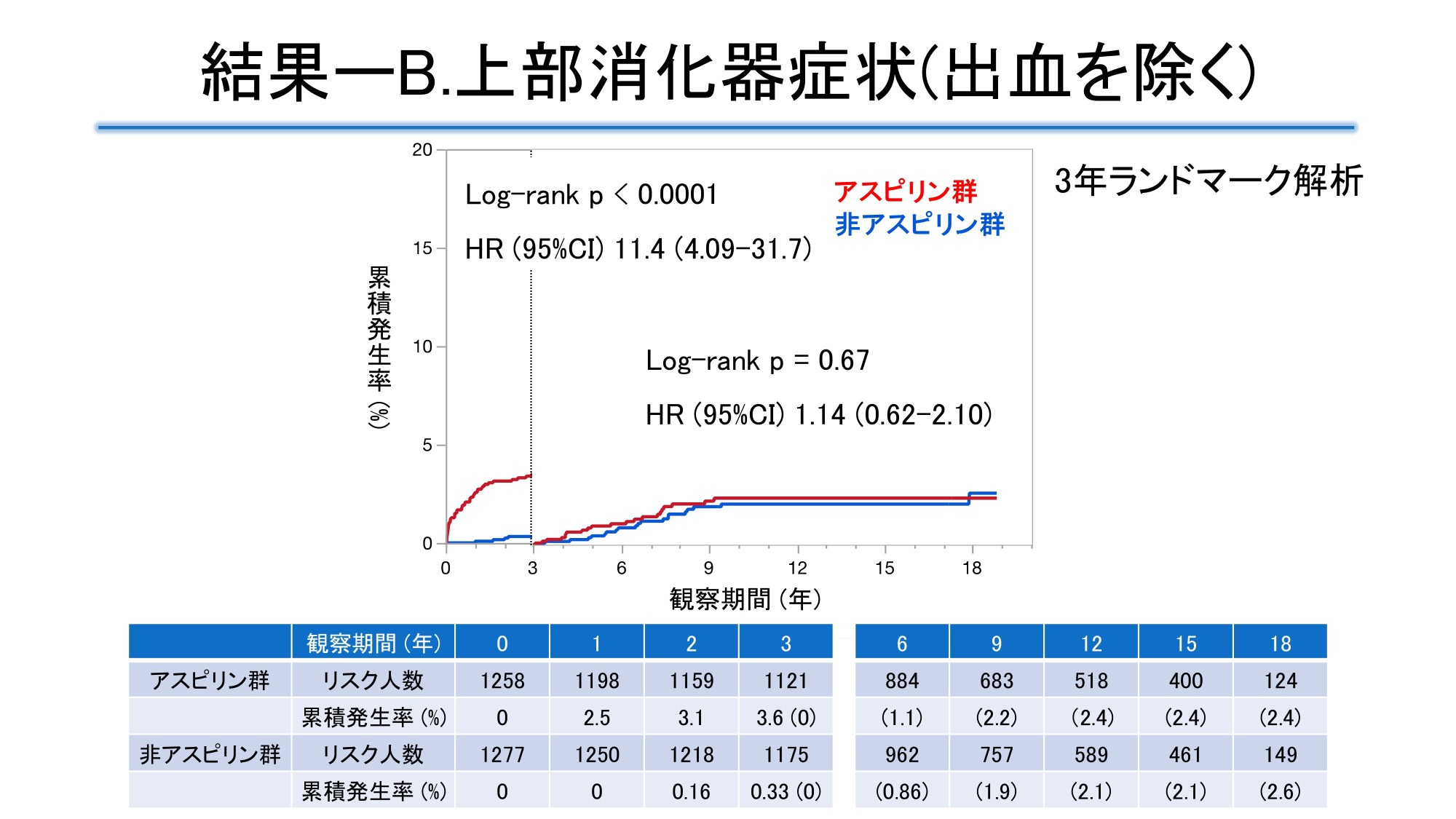
The mean age at enrollment was 65 years, 55% were male, and the median duration of diabetes was 7 years. At 18 years, the cumulative incidence of upper gastrointestinal events was 8.8% in the aspirin group and 5.7% in the non-aspirin group. Landmark analysis at 3 years showed that the HR in the aspirin group was 7.1 [95% CI 3.2-15.7] within 3 years, but 1.20 [95% CI 0.76-1.89] after 3 years, with the effect significantly weakening at the 3-year mark. For upper gastrointestinal symptoms other than bleeding, the HR in the aspirin group was 11.4 [95% CI 4.09-31.7] within 3 years, but 1.14 [95% CI 0.62-2.1] after 3 years. The event incidence rates for upper gastrointestinal bleeding and all bleeding events were low and not significantly different. When comparing the buffered tablet group and the enteric-coated tablet group in the aspirin group, the adjusted HR for upper gastrointestinal events in the enteric-coated tablet group within 3 years was 0.39 [95% CI 0.21-0.73], indicating a significantly lower risk of occurrence than in the buffered tablet group.
Future challenges
By following up on the randomized clinical trial over the long term even after the trial ended, we were able to obtain new findings that we had not anticipated. Since the clinical trial ended, it is possible that the treatment changed from the original randomization, but only 15% of the patients changed their assigned treatment (Circulation 2017;135:659). These results are likely to be useful not only for patients who are about to start taking low-dose aspirin, but also for patients who are currently taking it to decide what to do in the future.
In conventional risk factor analyses and guidelines, treatment selection is generally made by taking into account the risks at the time of starting treatment. However, because it has been shown that long-term observation like this can change the cohort and risk, similar long-term observation is also required for other preventive medical treatments that require long-term management.
Source of research funds
none
Publication information
Publication
American Journal of Cardiovascular Drugs (Impact Factor: 2.8)
Paper title
Long-term effects of low-dose aspirin on gastrointestinal symptoms and bleeding complications in patients with type 2 diabetes
"Long-term effects of low-dose aspirin on gastrointestinal symptoms and bleeding complications in patients with type 2 diabetes"
Author of the paper
Naoko Masutani (6th year Department of Medicine student Hyogo Medical University School of Medicine), Hisao Ogawa (President of Kumamoto University), Hirofumi Soejima (Associate Professor at Kumamoto University), Jounori Okada (Lecturer at Nara Medical University), Izuru Masuda (Researcher at Kyoto Medical Center), Masako Waki (Member of the Food Safety Commission of the Cabinet Office), Hideaki Jinnai (Chair of the Board of Trustees of Jinnai Hospital), Yoshihiko Saito (President of Nara Prefectural Seiwa Medical Center), Tsuyoshi Morimoto (Chief Professor Hyogo Medical University)
Furthermore, Masutani participated in the research doctor course from an early age, which helped him develop basic research skills and also contributed to this success.
Inquiries regarding this matter
総務部広報課
電話番号:0798-45-6655
E-mail:kouhou@hyo-med.ac.jp