Achievements
Development of a new heart failure treatment that activates a kinase expressed in cardiomyocytes
A research group led by Professor Tsukamoto Kura of the Department of Department of Biochemistry Hyogo Medical University Medicine (Location: Nishinomiya City, Hyogo Prefecture; President: Suzuki Keiichiro), in collaboration with the Department of Medical Chemistry, Osaka University Graduate School of Medicine (Graduate Student Hitotsumoto Tatsuro, Professor Takashima Seiji) and the Drug Discovery Center, Graduate School Graduate School of Pharmacy Osaka University (Specially Appointed Professor Haruta Junichi), has demonstrated that a decrease in the activity of myosin regulatory light chain kinase (cMLCK) causes dilated cardiomyopathy in humans, and has succeeded in developing a cMLCK activator as a new type of heart failure treatment.
*A paper on the results of this research was published in the electronic version of the American scientific journal Circulation on Tuesday, May 2, 2023, Japan time.
Topic
Restoration of cardiac myosin light chain kinase ameliorates systolic dysfunction by reducing super-relaxed myosin
Author of the paper
Hitotsumoto T, Tsukamoto K, Matsuoka K, Junjun Li, Li Liu, Kuramoto Y, Fujino A, Yoshida S, Higo S, Ogawa S, Kioka H, Kato H, Hakui H, Saito Y, Okamoto C, Inoue S, Hyedin Jo, Ueda K, Segawa T, Nishimura S, Asano H, Asanuma H, Tani A, Imamura R, Komagawa S, Kanai T, Takamura M, Sakata Y, Kitakaze M, Haruta J, Takashima S
Research Summary
In this study, we elucidated the mechanism by which human dilated cardiomyopathy develops due to a decrease in the activity of myosin regulatory light chain kinase (cMLCK), which is specifically expressed in cardiomyocytes. Furthermore, we succeeded in developing a small molecule compound that specifically activates cMLCK, and demonstrated that administering this compound to cardiomyocytes with decreased cMLCK activity reactivates cMLCK and restores contractility.
The effect of improving myocardial contractility by activating cMLCK is expected to make this a new treatment for heart failure that can safely improve hemodynamics while avoiding the serious side effects that were a concern with conventional inotropic drugs.
Research Background
cMLCK is a kinase that is specifically expressed only in cardiac myocytes, and it is already known that it can "physiologically control cardiac contractility" by phosphorylating cardiac myosin regulatory light chain. However, its involvement in the pathology of heart failure in humans was unknown.
In recent years, several research groups, including ours, have discovered several familial dilated cardiomyopathy cases with mutations in the MYLK3 gene that encodes cMLCK, and we hope to elucidate the molecular mechanism by which cMLCK activity affects the pathogenesis of heart failure and to identify the possibility of it becoming a new therapeutic target molecule. Conventional inotropes used in the treatment of severe heart failure can improve myocardial contractility by increasing intracellular calcium concentration. On the other hand, conventional inotropes have clinical issues such as side effects such as ischemia, arrhythmia, and sudden death. Against this background, there is a demand for the development of new types of inotropes that do not depend on the enhancement of intracellular calcium dynamics.
Research Methods and Results
In this study, our research group created mice and iPS-derived cardiomyocytes carrying the MYLK3 mutation identified in familial dilated cardiomyopathy, and analyzed the molecular mechanism behind the onset of heart failure.
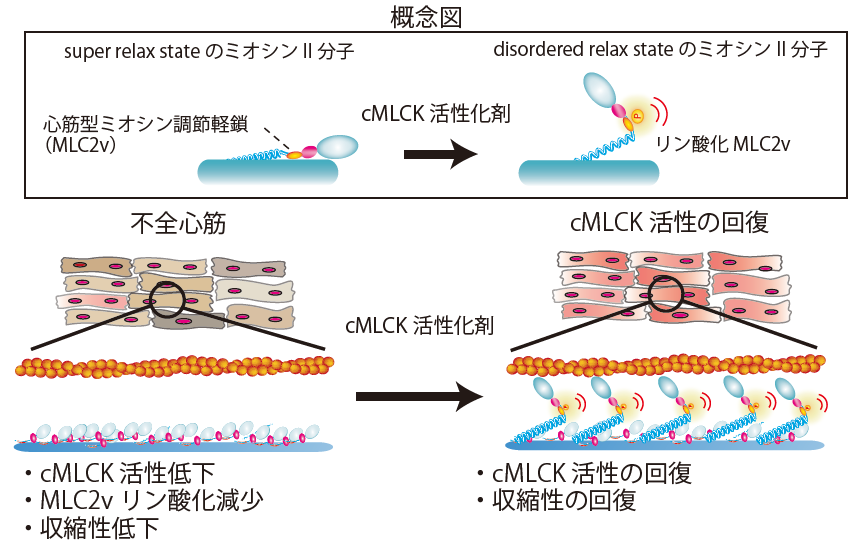
As a result, they found that when myocardial cMLCK activity decreased, the phosphorylation of cardiac myosin regulatory light chain decreased, the proportion of cardiac myosin II molecules in a so-called super relaxed state increased, and "myocardial contractility decreased."
Furthermore, by administering a proprietary cMLCK activator to iPS cardiomyocytes derived from patients with dilated cardiomyopathy with different causative genes, the researchers found that phosphorylation of myosin regulatory light chain was restored and the proportion of myosin II molecules in the super relaxed state was reduced, thereby improving myocardial contractility.
In addition, after examining myocardial tissue from patients with severe heart failure caused by different causative gene mutations, they discovered that "cMLCK activation was reduced" in the myocardial tissue of many patients with severe heart failure, regardless of the causative gene, revealing the possibility that cMLCK activators "may be effective in many patients with severe heart failure."
Future challenges
The "cMLCK activator" developed by our research group is able to improve contractility without changing intracellular calcium concentrations, and therefore may be a "heart failure treatment with a completely new mechanism" that avoids the side effects of conventional inotropic drugs and can safely restore hemodynamics in patients with severe heart failure.
In the future, we plan to confirm the therapeutic effectiveness in various heart failure models and examine whether early administration can prevent the onset and progression of heart failure pathology, with the aim of applying this treatment to clinical practice.
Source of research funds etc.
AMED Comprehensive Drug Discovery Support Program (Drug Discovery Booster)
Platform for supporting cutting-edge technologies such as drug discovery
Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research