About the Corporation
Clarification of the relationship between the risk of acute GVHD and blood ATG concentration in haploinsufficient transplantation
A research group led by Masahiro Teramoto, Assistant Professor, Department of Respiratory Medicine and Hematology, Hyogo Medical University School of Medicine (Location: Nishinomiya City, Hyogo Prefecture; President: Keiichiro Suzuki), conducted a study of patients with high-risk hematopoietic malignancies undergoing haplotransplantation. They found that blood ATG concentrations on the day of hematopoietic stem cell transplantation affect the risk of developing acute GVHD. Furthermore, blood ATG concentrations on the day of hematopoietic stem cell transplantation affected not only the incidence of acute GVHD, but also overall survival and relapse-free survival. Furthermore, simulations showed that by using the patient's ideal body weight when setting the ATG dosage, it is possible to optimize the dosage for each patient.
Topic
Individualized rabbit anti-thymocyte globulin dosing in adult haploidentical hematopoietic cell transplantation with high-risk hematologic malignancy: Exposure–response analysis and population pharmacokinetics simulations
Author of the paper
Masahiro Teramoto* (※1), Takuto Takahashi* (※2, 3), Kana Matsumoto (※4), Mutaz Jaber (※2), Katsuhito Kaida (※1), Hiroya Tamaki (※1), Kazuhiro Ikegame (※1), Satoshi Yoshihara (※1)
*Masahiro Teramoto and Takuto Takahashi are co-first authors.
*1 Department of Hematology Hyogo Medical University Hospital
*2 Pediatric Stem Cell Transplantation, Boston Children's Hospital, MA, USA
*3 University of Minnesota College of Pharmacy, MN, USA
*4 Clinical Pharmacology Laboratory, Doshisha Women's College of Liberal Arts
Research Summary
We have developed a method for HLA-matched allogeneic hematopoietic stem cell transplantation (haplotransplantation) (*2) that uses steroids and a small amount of rabbit-derived anti-human thymic immunoglobulin (ATG*1) to prevent acute graft-versus-host disease (GVHD), and have been working to improve the prognosis of patients with high-risk hematopoietic malignancies, such as those in remission. However, there has been insufficient research into optimizing the dosage of ATG for each patient.
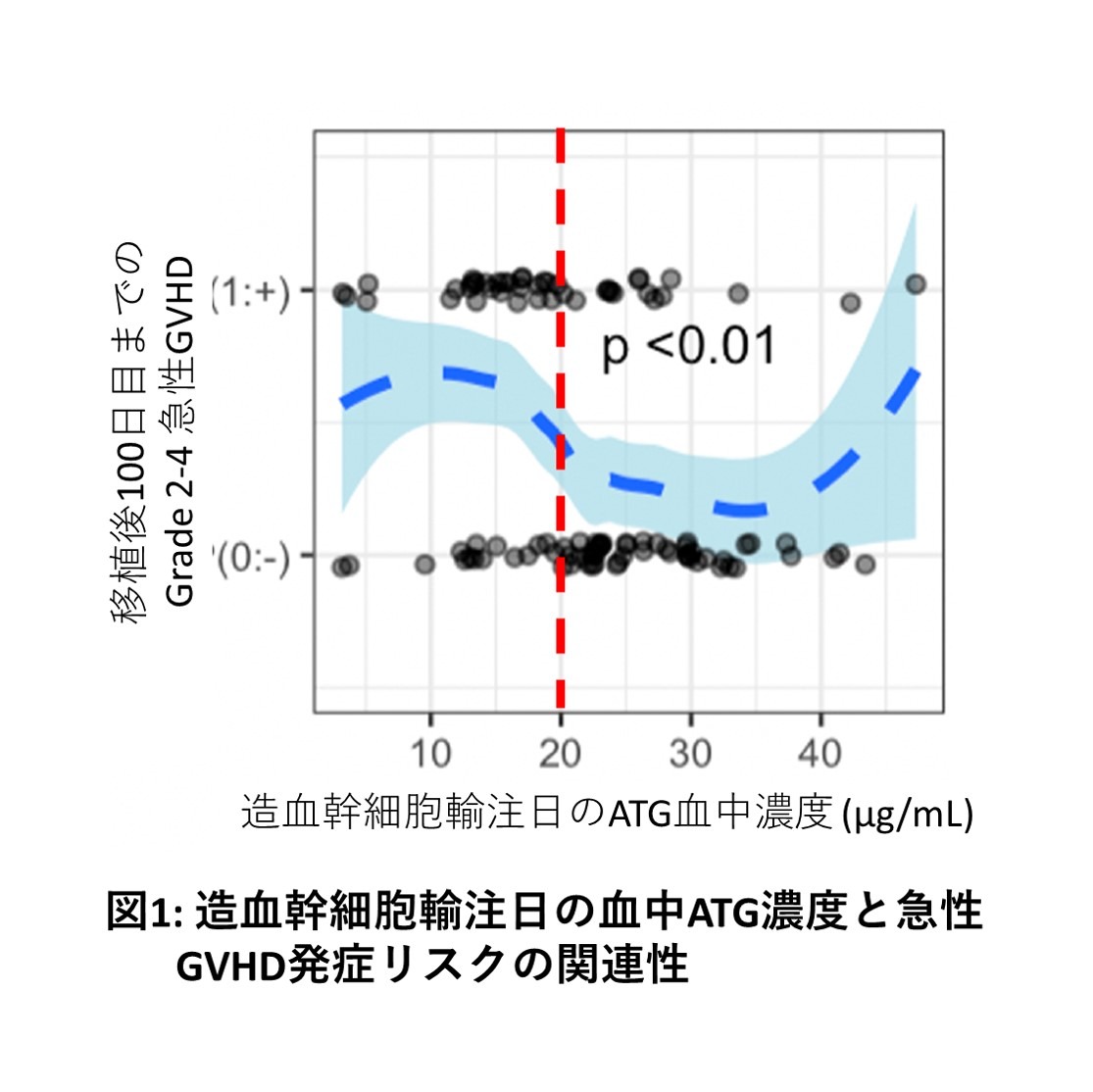
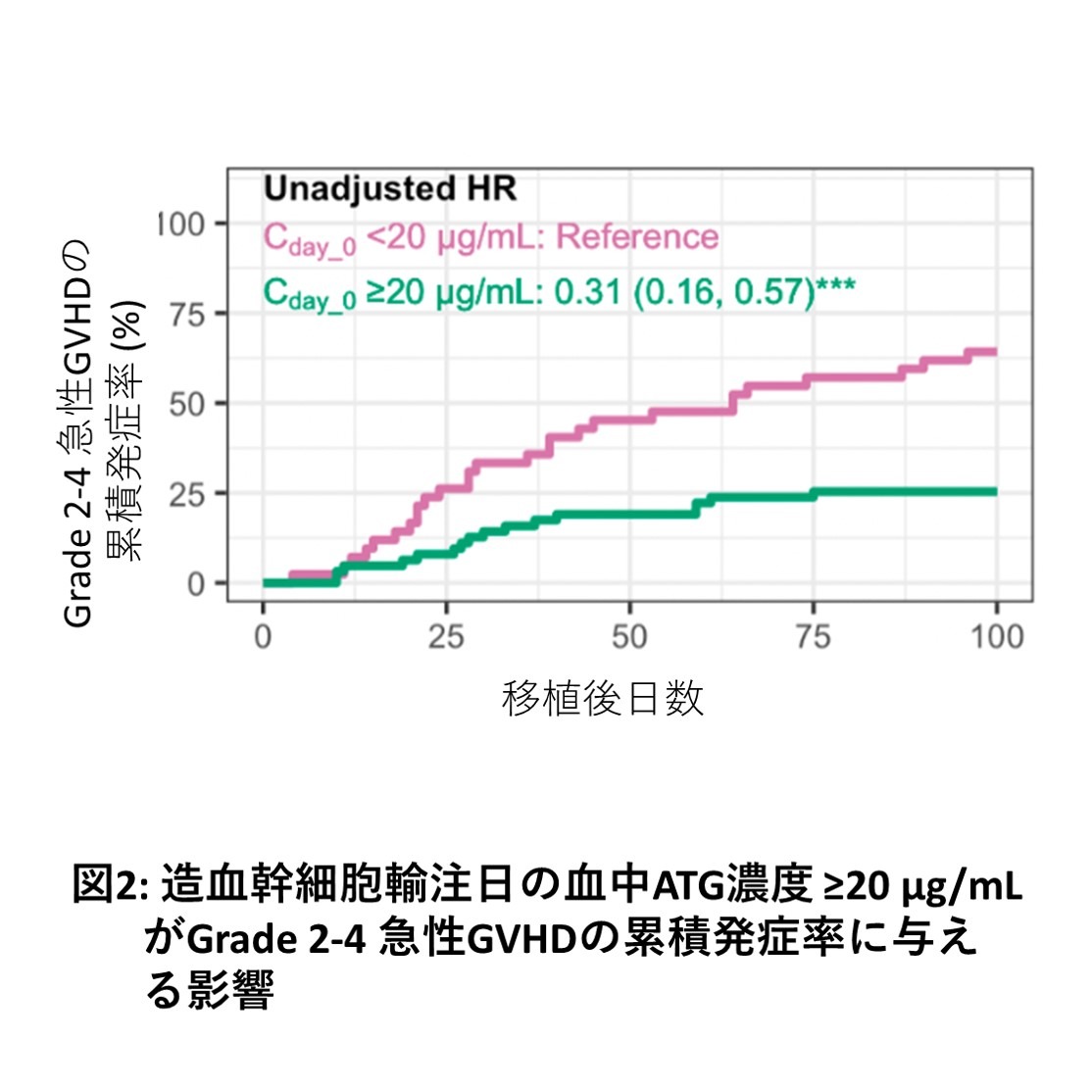
In this study, we used blood ATG concentration data from 103 patients with high-risk hematopoietic malignancies who underwent haplotransplantation between 2014 and 2019 to examine the impact of ATG concentration parameters on outcomes using exposure-response analysis and population pharmacokinetic models. Our results showed that a blood concentration of 20.0 µg/mL on the day of HSCT dichotomized the risk of Grade 2-4 acute GVHD, and that achieving a blood concentration of ≥20.0 µg/mL on the day of HSCT reduced the risk of acute GVHD (Figures 1 and 2). Furthermore, ATG dosage simulations indicated that setting an ATG dosage based on the patient's ideal body weight may facilitate achieving a blood concentration of ≥20.0 µg/mL on the day of HSCT. In the future, we plan to investigate whether individual optimization of ATG dosage is possible for allogeneic HSCT using ATG other than haplotransplantation.
Research Background
Our research group has previously been working on developing a haplotransplantation method for high-risk hematopoietic malignancies, such as those not in remission (Kaida K et al. Transplant Cell Ther 2023). Our haplotransplantation method is characterized by the use of steroids and a small amount of rabbit-derived ATG to prevent acute GVHD, a common complication of allogeneic hematopoietic stem cell transplantation. This method suppresses the severity of acute GVHD while also inducing a stronger antitumor effect from donor T cells. However, ATG is a drug with high inter-individual pharmacokinetic variability, making it difficult to determine an appropriate dose for each patient using conventional dosage planning based on the patient's actual body weight.
We received assistance from Dr. Takuto Takahashi of the Department of Clinical Pharmacology, School of Pharmacy, University of Minnesota/Department of Pediatric Hematopoietic Stem Cell Transplantation, Boston Children's Hospital, regarding the analysis of blood ATG kinetics, and from Dr. Kana Matsumoto of the Clinical Pharmacy Laboratory, School of Pharmacy, Doshisha Women's College of Liberal Arts, regarding the measurement of ATG blood concentrations, and we investigated the individual optimization of the ATG dosage for our haplotransplantation method.
Research Methods and Results
We retrospectively analyzed haplotransplantation cases for hematopoietic malignancies treated at our department between 2014 and 2019, in which steroids and low-dose ATG were used to prevent acute GVHD. Total ATG concentrations in patient serum were measured by ELISA (*3). Based on the obtained concentration data and patient clinical information, we performed an exposure-response analysis to examine the relationship between ATG blood concentration parameters and the onset of acute GVHD. We then determined the optimal target concentration parameter values for predicting acute GVHD. Furthermore, we performed simulations to develop an ATG dose calculation formula to achieve the target concentration parameter values. Because a previous study using population pharmacokinetic modeling confirmed that a patient's ideal body weight affects the pharmacokinetics of ATG (Takahashi T et al. Clin Pharmacokinet 2023), we decided to incorporate ideal body weight into the ATG dose calculation formula.
A total of 103 patients were analyzed, receiving a total of 2.5-3.0 mg/kg (actual body weight) of ATG before HSCT. Ninety-one patients (88%) underwent transplantation without remission of their underlying disease. In this patient population, the risk of developing Grade 2-4 acute GVHD was significantly dichotomized by a blood concentration of ≥20.0 µg/mL on the day of HSCT. Multivariate analysis of outcomes also showed that patients who achieved a blood concentration of ≥20.0 µg/mL on the day of HSCT had significantly better cumulative incidence of acute GVHD, overall survival, and relapse-free survival rates than those who did not. Therefore, in our haplotransplantation method, the target blood concentration of ATG to be achieved was determined to be ≥20.0 µg/mL on the day of HSCT. When ATG dosage was simulated based on this target value, a blood concentration of ≥20.0 µg/mL on the day of hematopoietic stem cell transplantation was achieved in 80% of simulations by administering a total of 3.0 mg/kg (ideal body weight) of ATG before hematopoietic stem cell transplantation.
Significance of this research result
This study used ELISA to measure ATG blood levels and clarified the relationship between ATG blood levels and the risk of acute GVHD. It also showed that in haplotransplantation for high-risk hematopoietic malignancies, the blood ATG levels on the day of hematopoietic stem cell transplantation affect the incidence of acute GVHD, overall survival, and relapse-free survival. Setting the ATG dosage based on ideal body weight may reduce the risk of acute GVHD in haplotransplantation using steroids and low-dose ATG, potentially improving the survival of patients with high-risk hematopoietic malignancies.
Future challenges
The ATG dosage design process in this study is only suitable for our department's haplotransplantation method. ATG is also used in allogeneic hematopoietic stem cell transplantation other than haplotransplantation, so in the future we would like to investigate whether ATG dosage can be optimized for other transplant conditions.
Glossary
*1: Rabbit-derived anti-human thymus immunoglobulin (ATG)
(Thymoglobulin®)
It is an immunosuppressant that is expected to have the effect of suppressing donor lymphocytes (T cells) primarily in the graft.
*2: HLA-matched allogeneic hematopoietic stem cell transplant (haplotransplant)
Haplotransplantation is an allogeneic hematopoietic stem cell transplant from a donor who is only half-matched with the human leukocyte antigen (HLA). It has the advantage of being easier to find a donor than a fully HLA-matched allogeneic hematopoietic stem cell transplant. However, due to the large degree of HLA incompatibility, it is important to note that the risk of severe acute GVHD is higher than with a fully HLA-matched allogeneic hematopoietic stem cell transplant.
*3: ELISA
Enzyme-Linked Immunosorbent Assay
This method utilizes the principle of antigen-antibody reaction to detect target components. It is used to quantify proteins, cytokines, and hormones, and to detect pathogens.
Source of research funds etc.
None