About the Corporation
Aiming to develop an inexpensive and effective innovative angiogenesis therapy using cell therapy - Investigator-initiated clinical trial using a combination of autologous peripheral blood mononuclear cells and injectable cell scaffold (ICS-001) for patients with comprehensive chronic limb ischemia (CLTI) -
Hyogo Medical University (Nishinomiya City, Hyogo Prefecture; President: Keiichiro Suzuki), Osaka Municipal University, Kinki University, Sofsera Co., Ltd., Gunze Medical Co., Ltd., and the Kobe Biomedical Innovation Center (TRI) with support from the Translational Research Program of the Japan Agency for Medical Research and Development (AMED) (Translational Research Support Organization: Okayama University, a National University Corporation; hereafter "Okayama University Center"), submitted a clinical trial notification in August of this year and initiated an investigator-initiated clinical trial Hyogo Medical University Hospital (Hospital Director: Masafumi Sakagami) using a combination of autologous peripheral blood mononuclear cells and the transplantation cell scaffold ICS-001 in patients with comprehensive severe chronic limb ischemia (CLTI) and intractable ulcers resistant to existing treatments.
This clinical trial will involve six planned cases to obtain clinical Proof of Concept (POC) for ICS (first-in-human confirmation of safety and estimation of efficacy). This exploratory clinical trial will be completed within the next three years, and after future confirmatory clinical trials, the aim is to obtain manufacturing and marketing approval for ICS as an angiogenic therapy for critical limb ischemia with intractable ulcers resistant to existing treatments.
1. Key points of this clinical trial
[Challenges and expected effectiveness/effects]
- Challenges with previous cell therapies aimed at angiogenesis: For CLTI that is resistant to existing revascularization procedures (endovascular therapy and bypass surgery), cell transplantation of autologous bone marrow or peripheral blood mononuclear cells has been developed for the purpose of angiogenesis, but its effectiveness has not been fully demonstrated.
- Improving angiogenic effects through cell therapy: By combining with ICS, we will develop a minimally invasive treatment that allows transplanted cells to remain in ischemic tissue and improves angiogenic effects.
- Provision of a new, minimally invasive, effective angiogenic cell therapy: By combining minimally invasive autologous peripheral blood mononuclear cells with ICS, we will provide an effective angiogenic cell therapy for CLTI that is resistant to existing treatments.
- Use of autologous peripheral blood mononuclear cells containing a large number of CD34-positive cells, which have a high angiogenic effect: The peripheral blood mononuclear cells used in combination with ICS this time will induce more CD34-positive cells, which have angiogenic effects, from the bone marrow. In order to do so, the peripheral blood mononuclear cells will contain G-CSF preparations (filgrastim [Gran®️]) and plerixafor (Mozobil®️), which are already in clinical use as a method for inducing hematopoietic stem cells such as CD34-positive cells into the peripheral blood.
- Development of cell therapy in the low-cost medical device category: By developing ICS as a medical device rather than as a regenerative medicine product, which can be expensive, it will become possible to provide inexpensive angiogenic cell therapy.
[Strong development system]
・Investigator-led clinical trials at Hyogo Medical University Hospital with the support of many universities and development companies
・Clinical trial coordination office run by the Medical Innovation Promotion Center (TRI) of the Kobe Biomedical Innovation Center, which has extensive experience in angiogenesis therapy using cell therapy
・Strong development support from AMED's Translational Research Program (Okayama University base)
2. Background of this research
Although angiogenesis therapy using cell transplantation for CLTI has been developed, its effectiveness is still insufficient. When CLTI is caused by arteriosclerosis obliterans, which is the common cause of the majority of cases, the standard treatment of revascularization, especially endovascular treatment, is highly effective in the short term, but it is thought that this is due to the difficulty in evaluating the effectiveness of angiogenesis therapy at the capillary and arteriole level.
On the other hand, the angiogenic effect of cell transplantation is thought to be due more to the indirect contribution of cytokines secreted by the transplanted cells than to the direct contribution of the transplanted cells themselves to form new blood vessels. However, it has been reported that most transplanted cells, such as mononuclear cells, quickly migrate into the systemic circulation and rarely remain in the ischemic tissue. Therefore, a dramatic improvement in the angiogenic effect can be expected by allowing the transplanted cells to remain in the ischemic tissue for a long period of time.
Aiming to improve angiogenesis therapy using cell transplantation, we have developed a substrate called ICS-001 that can be attached to transplanted cells and transplanted. By combining the low-cost ICS-001 with peripheral blood mononuclear cells, inexpensive cell therapy becomes possible.
As a result of previous research and development, we have obtained non-clinical proof of concept for ICS and completed non-clinical safety evaluations. We have now filed a clinical trial notification and begun an exploratory investigator-initiated clinical trial aimed at establishing angiogenesis therapy using ICS-001.
3. Target diseases
CLTI with intractable ulcers resistant to existing treatments
4. About this clinical trial
[Clinical trial project name]
An exploratory study of angiogenic therapy using autologous peripheral blood mononuclear cell-loaded ICS-001 transplantation for patients with refractory ulcers and CLTI
[Clinical trial identification code]
ICS-001
[Target patients and purpose]
The main objective of this study is to confirm the safety of angiogenic therapy using ICS-001 transplantation, which is carried by autologous peripheral blood mononuclear cells rich in hematopoietic stem cells (CD34 positive cells) collected by apheresis in combination with G-CSF preparation (filgrastim [Gran®️]) and plerixafor (Mozobil®️), in patients (6 cases) with comprehensive severe chronic limb ischemia (CLTI) and intractable ulcers resistant to existing treatments.
[Implementation Structure]
Clinical trial medical institutions and those who conduct the clinical trial themselves (principal investigators)
Kenichi Yamahara, Department of Hematology, Hyogo Medical University Hospital
Clinical Trial Coordination Office
Kobe Biomedical Innovation Center (TRI), a public interest incorporated foundation
Investigational Device Provider
Sofsera Co., Ltd.
Monitoring and Auditing
Okayama University Hospital New Medical Research and Development Center
Investigational Device Evaluation
Osaka Municipal University, Kinki University, Gunze Medical Co., Ltd.
Statistical analysis and data management
Kobe Biomedical Innovation Center (TRI), a public interest incorporated foundation
5. Reference diagram
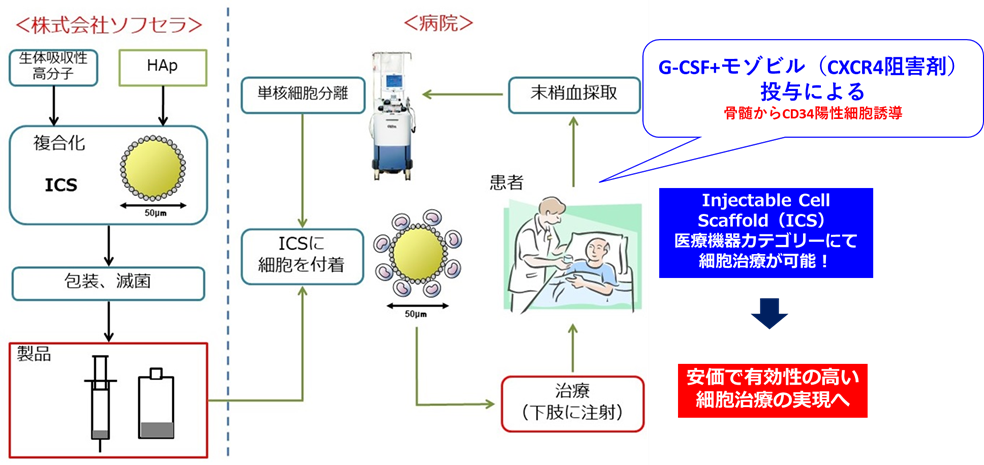
Figure 1: Cell therapy for critical limb ischemia in the medical device category
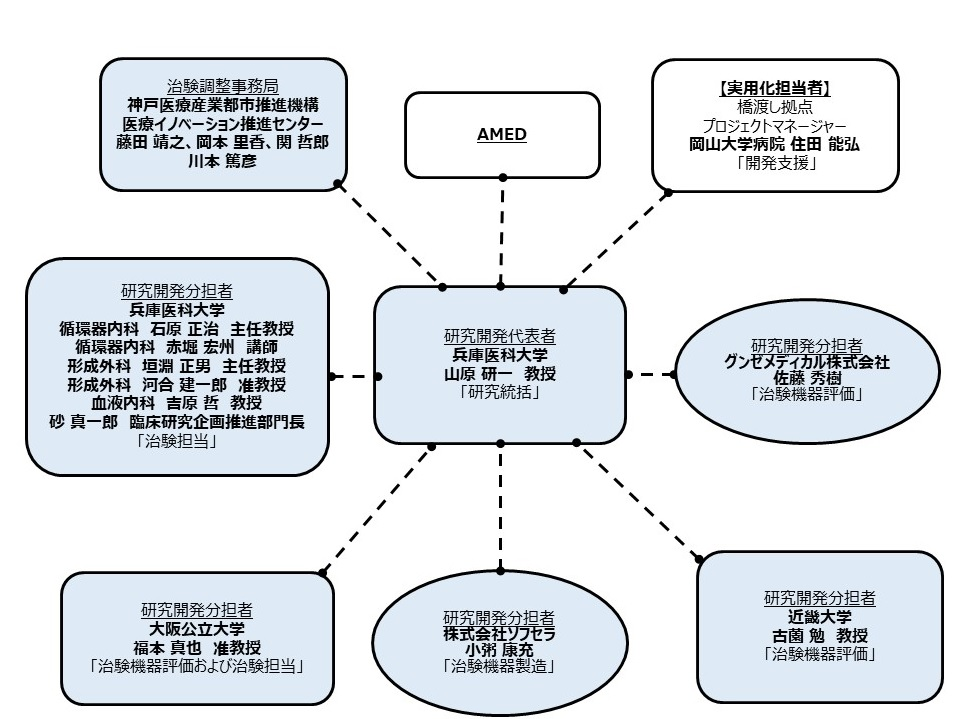
Figure 2: Research implementation structure
6. Notes
* Injectable cell scaffold (ICS)
ICS has a spherical structure with a diameter of approximately 50 μm, with a bioabsorbable polymer core and a surface coated with hydroxyapatite (HAp) single crystals. HAp has cell adhesion properties, allowing transplanted cells to be supported on its surface, and by retaining the transplanted cells in ischemic tissue for a long period of time, it exerts a strong angiogenic effect.
* Investigator-led clinical trial
A clinical trial (conducted to obtain approval under the Pharmaceuticals and Medical Devices Act) in which doctors themselves, rather than pharmaceutical companies, handle all tasks and operations, from planning to implementation and reporting.
* Regarding grants related to this research
Japan Agency for Medical Research and Development (AMED) Translational Research Program Seeds C(b) (FY2023-FY2025 (planned))
7. Related Patents
Patent No. 5725489
Invention title: Medical composition and kit
Patent holder: Osaka Municipal University, National Cerebral and Cardiovascular Center
Patent No. 5463504
Title of invention: Non-spherical microparticles and method for producing non-spherical microparticles
Patent holders: National Cerebral and Cardiovascular Center, Gunze Limited, Sofsera Co., Ltd.
Inquiries regarding this release
[Inquiries regarding research]
Hyogo Medical University School of Medicine Institute for Advanced Medical Sciences Laboratory of Molecular and Cellular Therapy
Professor Kenichi Yamahara
Phone: 0798-45-6398
[Press inquiries]
Hyogo College Hyogo Medical University General Affairs Department Public Relations Division
Phone: 0798-45-6655 Fax: 0798-45-6219
Email: kouhou@hyo-med.ac.jp
Osaka Public Relations Department, Osaka Municipal University
Phone: 06-6605-3411 Fax: 06-6605-3572
E-mail: koho-list@ml.omu.ac.jp
Kinki University Wakayama Campus Student Center Personnel: Nakai, Yamamoto, Nagai, Kanzaki
Phone: (0736) 77-3888 Fax: (0736) 77-7011
E-mail: bost-pr@waka.kindai.ac.jp
Sofsera Co., Ltd.
Phone: 072-641-8669 Fax: 072-646-8667
Email: kogai@sofsera.co.jp
Gunze Medical Co., Ltd. Corporate Planning Office
Phone: 06-4796-3151 Fax: 06-4796-3150
Contact: https://gunzemedical.co.jp/toiawase
Kobe Biomedical Innovation Center (TRI) Research consultation and support
Phone: 078-306-1015 Fax: 078-303-9098
Email: sodan@fbri.org
Okayama University, National University Corporation, General Affairs and Planning Department, Public Relations Division
Phone: 086-251-7292 Fax: 086-251-7294
E-mail: www-adm@adm.okayama-u.ac.jp