Tumor therapeutic agents and conjugates
Information updated: November 25, 2024
- Seeds Information
- Researcher Information
- What do you expect from collaboration with companies?
- Contact for this research
Seeds Information
keyword
Sphingosine-1-phosphate
Field
Breast cancer, solid cancer in general
Overview
Sphingosine-1-phosphate (S1P) is a lipid mediator with diverse physiological activities, and in tumor cells, the S1P signaling pathway has been reported to be involved in tumor cell proliferation, migration, survival, etc. One S1P receptor modulator, FTY720 (Fingolimod), is covered by health insurance in Japan as a treatment for multiple sclerosis, and has been shown to effectively suppress cancer proliferation and metastasis in in vitro experimental systems. In addition, three S1P receptor modulators (Ozanimod, Sponimod, Ponesimod), newly approved by the FDA as treatments for multiple sclerosis, are also expected to have the same cancer proliferation inhibitory effect as FTY720.
However, S1P receptor modulators such as FTY720 exert a strong immunosuppressive effect in vivo, which causes side effects on normal cells. Furthermore, the immunosuppressive effect of S1P receptor modulators contradicts one of the methodologies of cancer treatment, which is to release antitumor immunity against cancer cells.
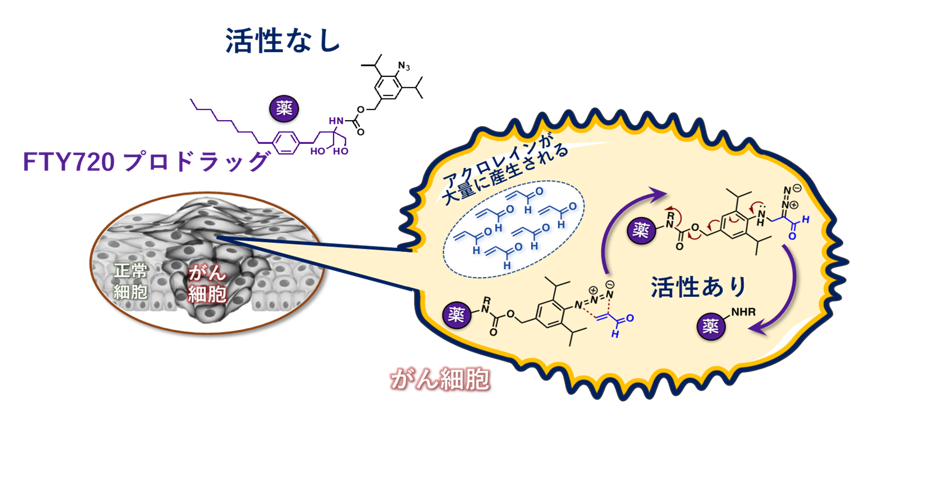
The present invention makes it possible to provide a novel prodrug that can act only on cancer cells by adding an azide probe (a probe with an azide group, which consists of three nitrogen atoms arranged in a line) as an acrolein reactive site, which was developed by Dr. Katsunori Tanaka, a senior researcher at the RIKEN, to an S1P receptor modulator such as FTY720.
What's new?
The present invention makes it possible to provide a novel prodrug that can act only on cancer cells by adding an azide probe (a probe into which an "azide group" consisting of three nitrogen atoms arranged in a line) as an acrolein reactive site to an S1P receptor modulator such as FTY720.
Until now, drugs targeting the S1P signaling pathway have had the problem of immunosuppression as a side effect, making them unsuitable as anticancer drugs. However, the present invention makes it possible to avoid immunosuppression by using a novel DDS, potentially realizing anticancer therapy targeting S1P for the first time.
What are its advantages over other studies?
The drug of the present invention has a mechanism for controlling (locational and functional) the release of the active drug by acrolein, which is produced in high concentrations specifically in cancer cells in correlation with the degree of malignancy after being taken up into the cell by endocytosis or the like, and has high selectivity against cancer. It has advantages in terms of controllability of release and broad range of drug applications over conventional FTY720 prodrugs, such as prodrugs PEGylated with a cyclic ketal group that is cleaved by acid (University of California) and prodrugs containing a self-cleavable linker (Acendis Pharma AS).
What problem does it help solve?
Because the mechanism of action of this newly developed drug is completely different from that of conventional antitumor drugs, it is expected to be effective against intractable cancers that are difficult to treat with conventional antitumor drugs. It is also expected to have a synergistic effect with existing therapeutic drugs, making it suitable for a variety of clinical applications.
Possibility of other applications and developments
The DDS of the present invention is applicable to compounds having an amino group, a hydroxyl group, or a carboxyl group, and is applicable not only to FTY720 but also to all S1P modulators, such as siponimod, ozanimod, and ponesimod, and may be applicable and developed to a variety of S1P agonists.
Related Patents
Tumor therapeutic agent and complex (Application number: PCT/JP2024/17295, Application date: May 9, 2024) (joint application with RIKEN)
Related papers
- Nagahashi M, Miyoshi Y. Targeting Sphingosine-1-Phosphate Signaling in Breast Cancer. International Journal of Molecular Sciences. 2024 Mar 15;25(6):3354.
- Nagahashi M, Abe M, Sakimura K, Takabe K, Wakai T. The role of sphingosine-1-phosphate in inflammation and cancer progression. Cancer science. 2018 Dec;109(12):3671-8.
- Nagahashi M, Yamada A, Katsuta E, Aoyagi T, Huang WC, Terracina KP, Hait NC, Allegood JC, Tsuchida J, Yuza K, Nakajima M. Targeting the SphK1/S1P/S1PR1 axis that links obesity, chronic inflammation, and breast cancer metastasis. Cancer research. 2018 Apr 1;78(7):1713-25.
Researcher Information
| full name | Masayuki Nagahashi |
|---|---|
| Affiliation | School of Medicine Department of Breast and Endocrine Surgery |
| Specialization | Surgical Oncology |
| Collaborative Researcher | Yasuo Miyoshi |
| Related links | Course introduction website |
What do you expect from collaboration with companies?
The drug of the present invention has the potential for broad clinical application to breast cancer and other solid cancers, and we hope to work with pharmaceutical companies to bring this developed drug to practical use through clinical trials.
Contact for this research
兵庫医科大学 大学事務部 研究推進課
E-mail: chizai@hyo-med.ac.jp
Tel: 0798-45-6488

 Research Seeds Collection
Research Seeds Collection