Synthetic intermediates for producing 3-haloaranes and aromatic compound library utilizing the reactions
Information updated: July 31, 2023
- Seeds Information
- Researcher Information
- What do you expect from collaboration with companies?
- Contact for this research
Seeds Information
keyword
Aryne, benzyne, halogen, aromatic ring, polycyclic heterocycle
Field
Chemistry, Pharmacy
Overview
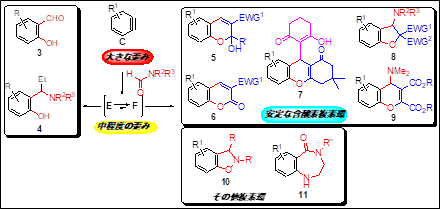
Arynes (1,2-benzyne or o-benzyne, simply called benzyne) are a general term for highly reactive chemical species that have a triple bond on an aromatic ring. Because of their high reactivity, arynes (C) are used in many fields, including the synthesis of biologically active substances and functional molecules such as polycyclic heterocycles that are difficult to synthesize using conventional methods.
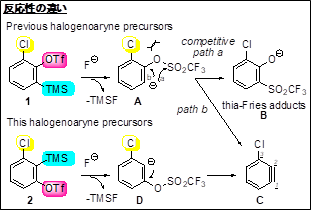
Conventional methods for synthesizing the precursor (1) of conventional aryne formation require a large number of experimental steps to selectively introduce or remove halogens at the desired positions via a halogen-lithium exchange reaction. It is also known that halogen-substituted six-membered carbon ring aromatic compounds generate arynes by eliminating halogen atoms in association with the generation of anions adjacent to the halogen atoms. For these reasons, there have been few systematic syntheses of halogen-substituted six-membered carbon ring aryne precursor compounds.
In addition, in the synthesis of various aromatic compounds via arynes, when an asymmetric aryne having a substituent is used as a substrate, two positional isomers may be generated, and therefore a method with higher reaction site selectivity has been required. For the purpose of controlling the reaction site, halogen-substituted arynes are effective, but the synthesis of the precursors by conventional methods is difficult.
The present aryne precursor (2) can be synthesized more easily and has a higher production efficiency than the conventional precursor (1). This research provides a precursor compound for generating 3-haloaryne (C), and also provides an aromatic compound library utilizing the reaction.
What's new?
- We succeeded in synthesizing various halogen-substituted aryne precursors in a simple manner with fewer steps.
- Achieved improved regioselectivity and reactivity of aryne reactions
What are its advantages over other studies?
- Reduces manufacturing costs compared to conventional align generation methods
- It is possible to synthesize compounds that cannot be derived from conventional aryne precursors.
What problem does it help solve?
Improved synthesis efficiency for the synthesis of functional molecules such as biologically active substances, pharmaceuticals, pharmaceutical intermediates, and electronic device materials (insulating films)
Possibility of other applications and developments
―
Related Patents
Synthetic intermediate for producing 3-haloarines and its synthesis method (Patent No. 6461914 (registered January 11, 2019))
Related papers
- (前駆体合成、従来との比較として)Regiocontrol Using Fluoro Substituent on 3,6-Disubstituted Arynes Chem. Pharm. Bull., 2023, 71, 775–781.
- (前駆体合成、従来との比較として)Aryne Precursors for Selective Generation of 3‑Haloarynes: Preparation and Application to Synthetic Reactions J. Org. Chem. 2020, 85, 13544−13556.
- (反応応用例として)Straightforward Synthesis of Dihydrobenzofurans and Benzofurans from Arynes Org, Lett., 2013, 15 (15), 3938-3941.
- (反応応用例として)A Multicomponent Coupling Reaction Induced by Insertion of Arynes into the C=O Bond of Formamide Angew. Chem. Int. Ed., 2011, 50 (29), 6638-6642.
Researcher Information
| full name | Eito Yoshioka |
|---|---|
| Affiliation | School of Pharmacy Fundamental Science |
| Specialization | Chemistry, Pharmacy |
| Collaborative Researcher | Hideto Miyabe |
| Related links | ― |
What do you expect from collaboration with companies?
Collaboration in research and development of pharmaceuticals, agricultural chemicals, chemical products, etc. based on this technology
Contact for this research
兵庫医科大学 大学事務部 研究推進課
E-mail: chizai@hyo-med.ac.jp
Tel: 0798-45-6488

 Research Seeds Collection
Research Seeds Collection