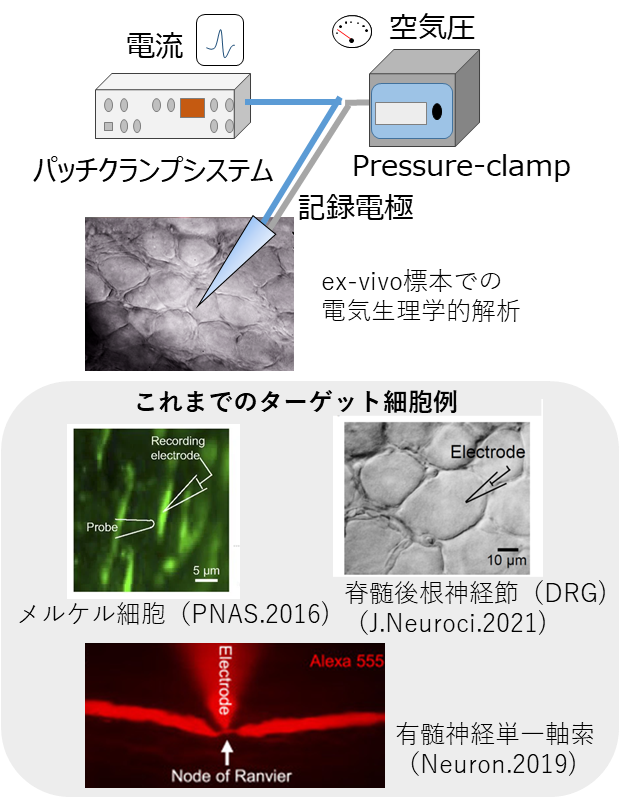
Pressure-clamp Analysis of physiological functions of various cells using patch clamp technique
Information updated: July 31, 2023
- Seeds Information
- Researcher Information
- What do you expect from collaboration with companies?
- Contact for this research
Seeds Information
keyword
Ion channels, Physiology, Department of Pharmacology
Field
Central, Pain
Overview
The patch clamp method is a method to directly measure the function of ion channels on the cell membrane. We have developed a pressure-clamp patch clamp method that incorporates a high-speed pressure-clamp (HSPC) device that controls the pressure inside the electrode into a conventional patch clamp system. By controlling the pressure inside the electrode, it is now possible to easily perform manipulations such as "breaking through connective tissue, cutting, peeling off cells, etc." on ex vivo specimens, which were not possible with the previous patch clamp method. Therefore, it has the advantage of being able to perform patch clamp on tissues and cells that have not been established as experimental systems until now. In addition, by delicately adjusting the pressure, we have succeeded in recording action potentials from ultrafine structures. Instead of Physiology evaluation in culture systems using various cell lines as in the past, it is possible to target various cells in ex vivo specimens of various organ tissues actually extracted from living organisms. We have applied this technology to Physiology analysis of various nerve cells, skin Merkel cells, Schwann cells, vascular endothelial cells, etc.
What's new?
Our unique technology (pressure-clamp patch-clamp method) enables manipulation of tissues, such as breaking through connective tissue, cutting, and peeling off cells, which was not possible with the conventional patch clamp method. This makes it possible to measure electrical activity from a variety of cells in various ex vivo specimens, including the nervous system and skin.
What are its advantages over other studies?
Until now, patch clamp techniques have been performed on cells in which specific genes have been forcibly expressed or on cells acutely isolated from living organisms. This technology uses excised living tissue as is, so there is no need for the previously required culture step, and patch clamp techniques can be performed under conditions that are structurally closer to physiological conditions. In addition, compared to in vivo experiments, it is easier to combine experiments with Department of Pharmacology or optogenetic/ Department of Genetics techniques.
What problem does it help solve?
The Physiology properties and function of ion channels can be directly determined for cell types that are not established as cell lines or are difficult to isolate from tissues, and functional analysis of gene-transfected cells can be performed directly in the tissue.
In addition to evaluating the functionality of specific cells, it is possible to visualize functional changes in various disease models or to perform Department of Pharmacology evaluations of drugs.
Possibility of other applications and developments
We have used this technique to develop patch clamp methods for various cells in ex vivo specimens, including nerve cells, nerve fibers, glial cells, vascular endothelial cells, skin cells, and supporting cells. By applying the technique to other organs, it will be possible to develop patch clamp methods or perform Physiology evaluations on a wide variety of cells.
Related Patents
―
Researcher Information
| full name | Hirosato Kanda |
|---|---|
| Affiliation | School of Pharmacy Pharmacotherapy |
| Specialization | Central, Pain |
| Collaborative Researcher | ― |
| Related links | ― |
What do you expect from collaboration with companies?
Joint research and technical guidance using the pressure-clamp patch-clamp method
Contact for this research
兵庫医科大学 大学事務部 研究推進課
E-mail: chizai@hyo-med.ac.jp
Tel: 0798-45-6488

 Research Seeds Collection
Research Seeds Collection